|
| ID | | Date | Severity | Status | Feedback |
| 10189562 | 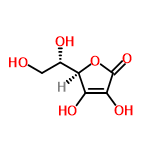 | 24/04/2024 07:05:05 | Extreme | New | Soy Gary. Mi objetivo como autor de artículos en la esfera de los
juegos de azar es proporcionar a los lectores información que les ayuden a conscientes en el mundo
de los juegos de azar. Intento ser neutral. aspectos del juego,
incluyendo tácticas de juego, reglas de varios juegos de
azar, acontecimientos reales y tendencias en la industria
como en https://CasteDoVascular.com.br/, así como recomendaciones
sobre control de riesgos y prevención de problemas de juego. |
| 4531960 | 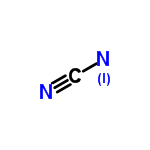 | 23/04/2024 12:18:37 | Normal | New | CN2 is not the molecular formula of the molecule pictured, which contains H2 |
| 59651624 | 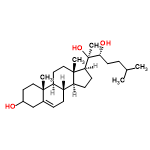 | 22/04/2024 13:57:40 | High | New | wiring a 7 way |
| 10189562 |  | 21/04/2024 05:53:10 | High | New | Top Shelf Trailers |
| 14088 | 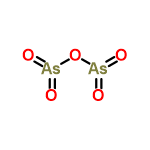 | 23/03/2024 14:02:35 | High | New | Name of this compound is Arsenic Pentoxide.
Thank You. |
| 2173494 | 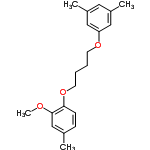 | 07/03/2024 21:26:27 | Normal | New | This compound has its info mixed up with another compound, Direct Blue 36.
They both seem to be associated with CASRN 6473-34-3 on multiple websites.
ECHA shows the blue compound for that CASRN, so I suspect that this page is the mis-entry.
|
| 4892458 | 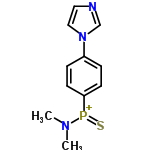 | 02/03/2024 16:04:33 | Normal | New | This compound is an incorrect structure for compound 20137954 |
| 4892462 | 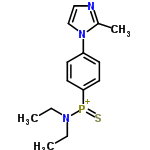 | 02/03/2024 15:50:24 | Normal | New | This structure (and the names) is wrong. The compound with RN 16914-02-06 is (+)-(2-Methyl-1H-imidazol-1-yl)phenyl(N,N-diethylamino)phosphine sulfide , i.e. the imidazole should be linked to P. This also fixes the valency issues. |
| 8156938 | 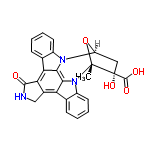 | 26/02/2024 13:29:43 | Normal | New | This chemical has been labelled as K252a, while the structure looks more like that of k252b (Pubmed CID 3814). There also seems to already be an entry labelled K-252a (ChemSpider ID2299962). |
| 14839071 |  | 23/02/2024 07:57:25 | Normal | New | I would like to purchase this compound: pyrrolopyrrole with the minimum amount. |
| 3083088 | 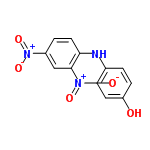 | 16/02/2024 12:16:37 | Normal | New | structure overlap, needs tidying B |
| 57269271 | 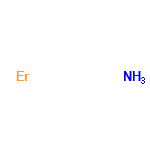 | 30/01/2024 21:00:47 | Normal | New | The RN number is wrong.
12020-21-2 is the number for Erbium nitride. So Erbium nitride ([Er].[N]) should be split off and get its own record with this CAS number. |
| 10482033 | 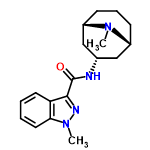 | 26/01/2024 15:59:46 | Normal | New | Surely Gransetron only has 2 chiral centres? Two of the ligands attached to the carbon of the amide bond are identical. |
| 34998836 | 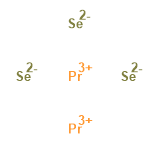 | 23/01/2024 00:41:58 | Normal | New | RN is wrong as the number here 12038-08-3 is for PrSe Praseodymium monoselenide. |
| 56442 | 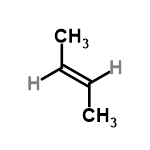 | 21/01/2024 12:04:46 | High | New | I can't find the IUPAC name of the structure I've drawn,which is a must to be present |
| 40272 | 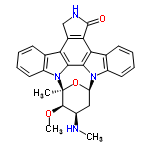 | 19/01/2024 12:30:53 | Normal | New | Dear Colleagues: Neither the borregomycins or the tasikamides are included as yet. Thank you. Prof Geoff Cordell |
| 66498 | 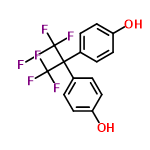 | 19/01/2024 09:12:43 | Normal | New | structure needs tidying - removing overlap B |
| 94547 |  | 15/01/2024 14:54:10 | Normal | New | Under the second header that is red. Organization is spelled incorrectly. |
| 57 | 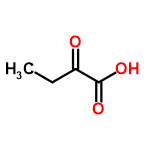 | 10/01/2024 19:19:43 | Normal | New | can't it also be 2-oxobutanoic acid? |
| 403789 | 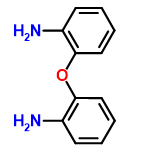 | 07/01/2024 09:34:42 | Normal | New | structure needs tidying |
|