SyntheticPage 304
Published Feb 12, 2009
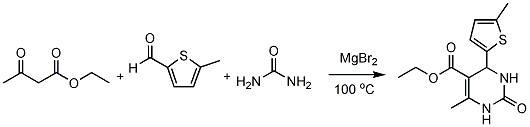
Procedure
A mixture of ethyl acetoacetate (2 mmol), 5-methylthiophene-2-carboxaldehyde (2 mmol), urea (3 mmol) and magnesium bromide (0.2 mmol) was heated at 100 oC with stirring until the mixture turned to solid mass (45 min). After completion (monitored by TLC), the solid was cooled to room temperature and poured onto crushed ice (20 g) and stirred for 10 min. The crude product was filtered, washed with cold water (2x10 mL) and then recrystallized from ethanol or ethyl acetate/n-hexane to afford white crystals. Yield (494 mg, 88%)
Full Article
SyntheticPage 489
Published Mar 25, 2011
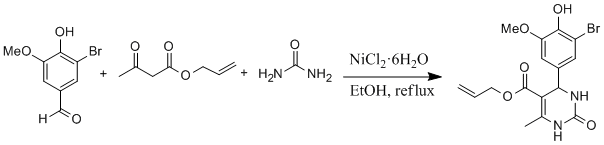
Procedure
A solution of allyl acetoacetate (2.5 mmol), 5-bromovanillin (2.5 mmol), urea (3.75 mmol), nickel chloride hexahydrate (0.62 mmol) and conc. HCl (2 drops) in EtOH (5 mL) were heated under reflux for 6h. After cooling to room temperature the reaction mixture was poured onto crushed ice (20 g) and stirred for 10 min. The crude product was filtered, washed with cold water (2x10 mL) and then recrystallized from ethanol afford white solid. Yield (0.74 g, 75%).
Full Article
SyntheticPage 826
Published Jun 21, 2017
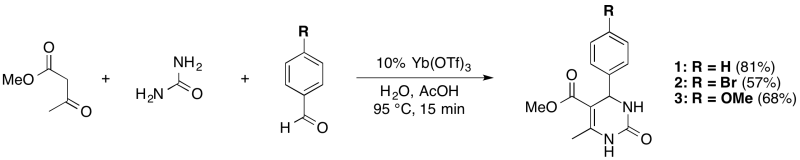
Procedure
Urea (0.48 g, 8.0 mmol) and ytterbium(III) triflate (0.26 g, 0.4 mmol) were added to a 1 dram (3.7 mL) glass reaction vial. Then, the 3:1 acetic acid:water solvent mixture (2 mL) was added to the vial; next, the methyl acetoacetate (0.86 mL, 8 mmol) was added, followed by the aldehyde (4 mmol). The stirred solution was heated for 15 minutes at 95 °C and developed an intense yellow colour. The reaction vial was cooled to room temperature; and, its contents were poured into a 25 mL beaker with 2 mL of ice and 5 mL of deionized water to fully precipitate the product. The solid was collected by vacuum filtration using a hirsch filter. The solid was washed with cold water (5 mL), then with toluene (5 mL); removal of the residual solvent by rotary evaporation provided the product in reasonable purity.
Full Article
 Ytterbium (III) triflate catalyzed Biginelli reactions
Ytterbium (III) triflate catalyzed Biginelli reactions