SyntheticPage 693
Published Sep 06, 2013
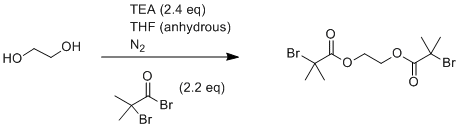
Procedure
Ethylene glycol (1.80 mL, 32.4 mmol) and an excess of triethylamine (9.90 mL, 71.3 mmol) were added into a 500 mL round bottom flask with a stirrer bar and flushed with nitrogen for 15 minutes on an ice bath. Anhydrous THF (150 mL) was added into the flask via an airtight syringe, and allowed to cool to 0 °C. Under a nitrogen atmosphere, α-bromoisobutyryl bromide (8.40 mL, 68.1 mmol) was added slowly via a dropping funnel. The reaction mixture was filtered in order to remove the triethylammonium bromide salt formed, and the solution was concentrated by rotary evaporation. The resulting yellow/brown solution was then stirred with 0.10 M aqueous sodium carbonate to hydrolyze any remaining 2-bromoisobutyryl bromide. The crude product was then extracted with dichloromethane (3 × 50 mL) using a separating funnel and the combined dichloromethane extracts were dried with anhydrous magnesium sulfate and then filtered. The solvent was removed via rotary evaporation, yielding yellow crystals upon cooling. The product was purified by flash column chromatography with 3:1 ethyl acetate:hexane to give white crystals (8.2 g, 70.3%)
Full Article
 Esterification of ethylene glycol with α-bromoisobutyryl bromide
Esterification of ethylene glycol with α-bromoisobutyryl bromide
 Esterification of ethylene glycol with α-bromoisobutyryl bromide
Esterification of ethylene glycol with α-bromoisobutyryl bromide