SyntheticPage 753
Published Aug 12, 2014
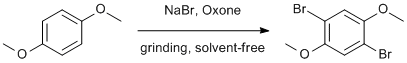
Procedure
1,4-dimethoxybenzene (0.56 g, 4.0 mmol) was placed in a mortar and pestle and sodium bromide (0.82 g, 8.0 mmol) was added. Oxone (2.45 g, 4.0 mmol) was added and the mixture was ground for approximately 15 min in the mortar until the reaction mixture had attained a uniform waxy texture. The solid was washed with water while grinding in the mortar, and collected on a fritted funnel. The product was recrystallized from 95% ethyl alcohol by transferring the solid to an Erlenmeyer flask and gradually heating with a heat gun while adding ethanol until the solid dissolved entirely. Slow cooling on the bench to room temperature followed by cooling in an ice bath gave white, needle-like crystals, which were collected by Buchner filtration and rinsed with ice-cold ethanol. (0.98g, 83% yield)
Full Article
SyntheticPage 789
Published Jul 21, 2015
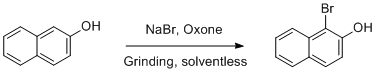
Procedure
0.58g (4 mmol) of 2-naphthol, 0.41g (4 mmol) of sodium bromide and 1.225g (2 mmol) of oxone were placed in a mortar and pestle. The reagents were ground together ca. 1-2 minutes until they became a fine powder, then transferred into a plastic reagent bottle with grinding media. (See Notes) The bottle was placed inside a rock tumbler container and tumbled for five hours or overnight. Dichloromethane (ca. 10 mL, see Notes) was added to the container, the container was sealed and vigorously shaken by hand, and the liquid was gravity filtered into a 100 mL round bottom flask through a fluted paper. The dichloromethane wash was repeated a total of 3 times, and the resulting dark brown-red solution was reduced to a solid by rotary evaporation. The resulting crude 1-bromo-2-naphthol is a dark brown solid showing flecks of iridescent color, approximately 0.7g. (See Notes)
This product was crystallized from boiling
n-heptane by transferring the solid to a 125 mL Erlenmeyer flask and adding ca. 10 mL of heptane, then heating to boiling using a heat gun. The hot solution was separated from the dark oily residue by decanting into a beaker pre-cooled in an ice bath, and the resulting clusters of beige, needlelike crystals were collected in a Büchner funnel and washed with ice-cold
n-heptane, yielding 0.383 g (1.72 mmol) of
1-bromo-2-naphthol, 43% yield.
Full Article
SyntheticPage 791
Published Jul 22, 2015
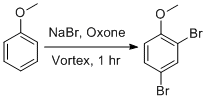
Procedure
Anisole (1.08g, 10 mmol), sodium bromide (3.6g, 35 mmol), oxone (10.72g, 17.5 mmol) were added together with approximately 20 copper-plated steel beads to a 50mL centrifuge tube on a vortex mixer and the mixture was vortexed for approximately 60 minutes. (See Notes) The contents of the tube were poured out onto a Buchner funnel and rinsed well with deionized water and the beads removed with a magnet. Air was pulled through the collected product for ca. 10 minutes to remove water and traces of bromine vapor, yielding 0.65 g dense white flakes of product. During this time, a second crop of crystalline product separates from the filtered reaction mixture and can be collected by a second vacuum filtration yielding 0.86 g solid for a total of 1.51 g (5.5 mmol, 55%) 2,4-dibromoanisole, >90% pure by GC/MS.
Full Article
 Solventless dibromination of 1,4-dimethoxybenzene
Solventless dibromination of 1,4-dimethoxybenzene