SyntheticPage 517
Published Nov 27, 2011
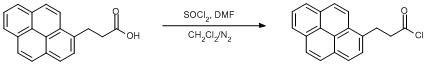
Procedure
3-(1-Pyrenyl)propionic acid (0.1 g, 0.36 mmol) was dissolved in DCM (15 ml) with stirring and placed in an ice bath (0oC) under a dinitrogen atmosphere. Thionyl chloride (0.026 ml, 0.36 mmol) was then added carefully and thereafter DMF (two drops). The reaction was left in the inert atmosphere for an hour at room temperature. The solvent was removed in vacuo and an orange crystalline solid is formed in high yield (96%).
Full Article
SyntheticPage 633
Published Jul 19, 2013
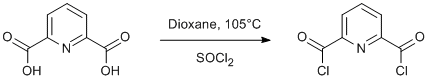
Procedure
Pyridine-2,6-dicarboxylic acid (18.3 g, 0.11 mol) and thionyl chloride (19 ml) were dissolved in dioxane (50 ml) and heated to reflux for 3 h at 105°C. The solvent and excess thionyl chloride were removed under reduced pressure. The residue was distilled under vacuum (180-190°C, 4x10-3 mbar). Yield 15.9 g (71%).
Full Article
SyntheticPage 886
Published Apr 17, 2019
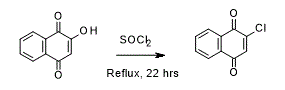
Procedure
To a round-bottomed flask was charged 2-hydroxy-1,4-napthoquinone (3.00 g, 17.2 mmol) and thionyl chloride (30 mL, 413.4 mmol), the reaction mixture was heated to reflux with constant stirring for 22 hours*. Upon cooling the reaction mixture was evaporated to dryness** and the dark yellow solid produced was washed with diethyl ether to yield 2-chloro-1,4-napthoquinone (2.84 g, 86%) as an orange solid.
Full Article
 Chlorination of a carboxylic acid
Chlorination of a carboxylic acid