SyntheticPage 467
Published Sep 30, 2010
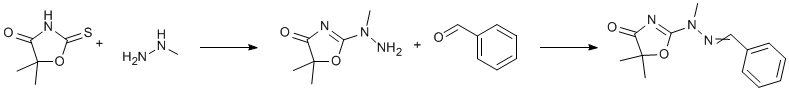
Procedure
Methylhydrazine (1.02 ml) was added to a solution of 5,5-dimethyl-4-oxazolidinone-2-thione (2.9 g) in methanol (15 ml). After 4 h, benzaldehyde (2.2 ml) was added. On seeding, the product separated. Next day, the solid was filtered off, washed with a little ethanol to afford 3.48 g (71%),
Full Article
SyntheticPage 477
Published May 17, 2011
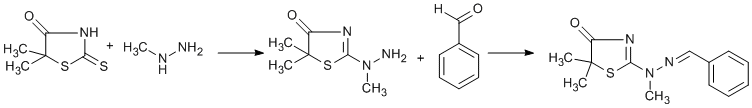
Procedure
5,5-dimethylrhodanine (1.3 g, 0.008mole) and methylhydrazine (0.8 ml, 0.015 mole) were stirred overnight in methanol (20ml). Next day, some sulphur which had separated was filtered off and benzaldehyde (1.6ml, 0.015 mole) was added to the filtrate.Next day the product (1.1 g, 52%) was filltered off and washed with a little ethanol.
Full Article
SyntheticPage 484
Published Feb 11, 2011
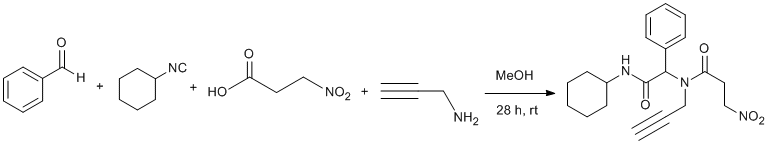
Procedure
A solution of benzaldehyde (1.70 mmol, 0.172 mL), propargylamine (1.70 mmol, 0.11 mL), cyclohexyl isocyanide (1.70 mmol, 0.211 mL) and 3-nitropropionic acid (1.70 mmol, 0.202 g) in 10 mL MeOH was stirred at room temperature under nitrogen atmosphere. After 28 h the solution was concentrated under reduced pressure to leave a yellow solid. The residue was washed with cold Et2O (3x20 mL) to give pure title compound as pale yellow solid (480 mg, 76% yield, mp 179-180 °C, Rf= 0.52 (ethyl acetate-hexane; 1 : 1). 1H and 13C NMR spectra showed title compound is a mixture of two rotamers in an approximately 1:3.5 ratio.
Full Article
SyntheticPage 485
Published Feb 14, 2011
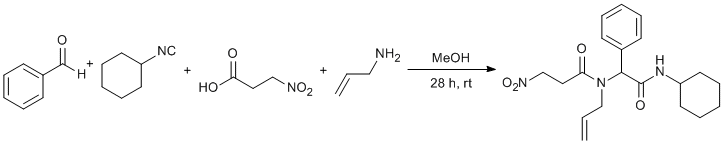
Procedure
A solution of benzaldehyde (1.70 mmol, 0.172 mL), allylamine (1.70 mmol, 0.128 mL), cyclohexyl isocyanide (1.70 mmol, 0.211 mL) and 3-nitropropionic acid (1.70 mmol, 0.202 g) in 10 mL MeOH was stirred at room temperature under nitrogen atmosphere. After 28 h, the solution was concentrated under reduced pressure to leave a pale yellow solid. The residue was washed with cold Et2O (3 x 20 mL) to give pure title compound as a white solid (475 mg, 75% yield, mp 190-192 °C, Rf= 0.44 (ethyl acetate-hexane; 1 : 1). 1H and 13C NMR spectra showed title compound is a mixture of two rotamers in an approximately 1:4.5 ratio.
Full Article
SyntheticPage 588
Published Feb 13, 2013
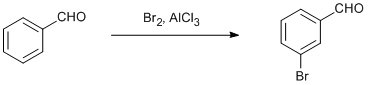
Procedure
To a clean 2 l 4 necked flask carrying calcium chloride guard tube, thermometer pocket and a mechanical stirrer, was charged Dichloroethane (500 mL) followed by anhydrous aluminium chloride (177 g, 1.32 mol) under stirring. To this benzaldehyde (104 g, 1 mol) was added over a period of 1 h at 38-40 oC. After this bromine (176 g, 56.8 mL, 1.1 mol) was added over a period of 2 h at about 40 oC and further the mixture was stirred at the same temperature for 2 h. The reaction mass was quenched to approximately 1 kg crushed ice and stirred for 10 min. The organic layer separated was removed and washed with water ( 1000 mL) and 300 mL sodium carbonate solution(5%) followed by water (750 mL). The organic layer thus obtained was concentrated to get about 200 g residue. This residue was distilled under vacuum to get 160 g of pure 3-bromo benzaldehyde (Yield: 87%).
Full Article
SyntheticPage 593
Published Apr 23, 2013
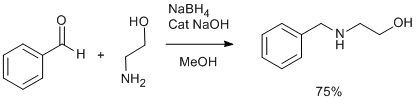
Procedure
A 1-liter 3 necked round bottomed flask containing a mechanical stirrer, thermometer pocket, water/oil heating bath was assembled under an atmosphere of dinitrogen. Ethanol (400 mL) and aminoethanol (61 g, 1 mol) were charged. The reaction temperature was raised to about 45 oC. To this solution, benzaldehyde (106 g, 1 mol) was added over a period of 30 min. The reaction mass was further refluxed for 3 h. At this point, TLC of the reaction mixture shows almost conversion of benzaldehyde to corresponding imine (2:8, ethylacetate: hexane). The reaction mass was cooled to about 35-40oC and a solution of sodium borohydride (19 g, 0.5 mol) in 40 mL aqueous sodium hydroxide solution (400 mg NaOH in 40 ml water) was added over a period of 90 min keeping the reaction temperature below 40oC. The reaction mixture was stirred at the same temperature for 4 h. The ethanol was distilled off almost completely under vacuum. The reaction mass was added to about 250 mL water containing ammonium chloride (50 g) and extracted with dichloromethane at least 4 times (500 mL each). The dichloromethane extracts were washed with sodium carbonate solution (500 mL, 10%), dried with sodium sulfate and evaporated thoroughly to leave an oily mass (140 g, 92.7%) about 95% pure by GC. This can be further purified by vacuum distillation (one can get about 75% main fraction). Alternatively, this can be purified by converting to maleate salt - please find details below. The maleate can be bascified and extracted in dichloromethane.
Purification of the product by converting into Maleate salt:
The crude product from above reaction (~ 140 g , 0.92 mol) was dissolved in ethyl acetate (800 mL ) by warming to about 50 oC. Maleic acid (107 g, 0.92 mol) was added all at once and the mixture was stirred for 15 min then cooled to 20 oC. The almost white solid was filtered off (~ 325 g, wet) and washed with ethyl acetate (200 mL). The solid thus isolated was added to 5 l flask containing water ( 900 mL) and dichloromethane (750 mL) under stirring. The mixture was chilled to 10oC and treated with aqueous ammonia (~200 mL) . The dichloromethane layer thus obtained was separated and the water layer extracted with further dichloromethane (750 mL). The combined organic extracts were washed with water ( 500 mL) and dried over sodium sulphate ( 200g). Finally, dichloromethane was removed under reduced pressure to get about 110-113 g (~ 75%) pure product.
Full Article
SyntheticPage 736
Published Apr 22, 2014
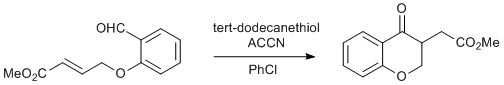
Procedure
To a solution of the benzaldehyde (200 mg, 0.91 mmol, 1 equiv.) in dry chlorobenzene (10 mL) were added ACCN (335 mg, 1.37 mmol, 1.5 equiv.) and tert-dodecanethiol (553 mg, 2.73 mmol, 3 equiv.) (Note 1). The solution was sparged by bubbling argon through the solution for 30 minutes after which the solution was maintained under an atmosphere of argon and heated at 100 °C for 20 hours. After removal of the solvent in vacuo (ensure adequate ventilation due to pungent thiol), the resulting oil was purified by column chromatography (2 x 25 cm column of silica gel, EtOAc-PE, 1 : 3) to give the benzopyran-4-one (136 mg, 68% [92% based on recovered benzaldehyde], Rf = 0.63 in EtOAc-PE, 1 : 1) and starting benzaldehyde (52 mg, 26%, Rf = 0.55 in EtOAc-PE, 1 : 1).
Full Article
SyntheticPage 771
Published Dec 21, 2014

Procedure
A solution of NaOH (0.902 g, 22.55 mmol, 1.6 eq) in distilled water (40 mL) at room temperature was added to a stirred solution of benzaldehyde (4.361 g, 41.13 mmol, 2.9 eq) in ethanol (40 mL). Acetone (0.833 g, 14.36 mmol, 1.0 eq) was added to the reaction mixture. The reaction mixture was then stirred at room temperature for 30 minutes. The precipitated product was recovered by suction filtration, washed 1x with 2-3mL of 1:1 ethanol/water, dried over suction for 25 minutes, and recovered (3.044 g, 90.6%) as yellow crystals (mp 103-104°C).
Full Article
SyntheticPage 787
Published Jul 10, 2015
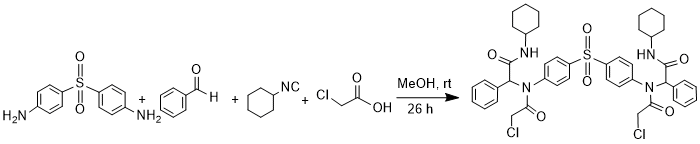
Procedure
A solution of 4,4'-diaminodiphenylsulfone (0.805 mmol, 200 mg) in MeOH (7 mL) was treated with benzaldehyde (1.61 mmol, 0.164 mL), a solution of cyclohexyl isocyanide (1.61 mmol, 0.197 mL) in MeOH (3 mL), and chloroacetic acid (1.61mmol, 152 mg) in the order given. The reaction mixture was stirred for 26 hours at room temperature, and then filtered. The precipitate was washed with first Et2O and then with n-hexane to remove unreacted reagents or by-products and dried to give white Ugi adduct (600 mg, 90%). m.p. 222-225oC, Rf(80%EtOAc/n-hexane): 0.82
Full Article
SyntheticPage 795
Published Aug 25, 2015
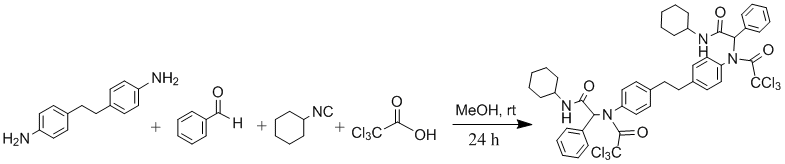
Procedure
A solution of 4,4'-diaminobibenzyl (0.94 mmol, 200 mg) in MeOH (7 mL) was treated with benzaldehyde (1.88 mmol,0.19 mL), a solution of cyclohexyl isocyanide (1.88 mmol, 0.23 mL) in MeOH (3 mL), and trichloroacetic acid (1.88 mmol, 310 mg) in the order given. The reaction mixture was stirred at room temperature for 24 hours, and then filtered. The precipitate was washed with first Et2O and then with n-hexane to remove unreacted reagents or by-products and dried to give white Ugi adduct (630 mg, 72%). m.p. 227-230oC, Rf(50%EtOAc/n-hexane): 0.77
Full Article
SyntheticPage 796
Published Aug 25, 2015
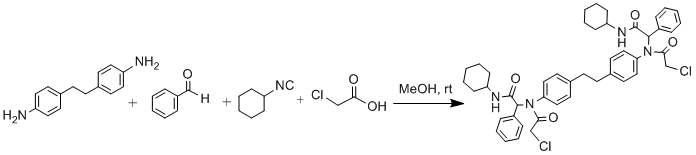
Procedure
A solution of 4,4'-diaminobibenzyl (0.94 mmol, 200 mg) in MeOH (7 mL) was treated with benzaldehyde (1.88 mmol, 0.19 mL), a solution of cyclohexyl isocyanide (1.88 mmol, 0.23 mL) in MeOH (3 mL), and chloroacetic acid (1.88 mmol, 178 mg) in the order given. The reaction mixture was stirred at room temperature for 24 hours, and then filtered. The precipitate was washed with first Et2O and then with n-hexane to remove unreacted reagents or by-products and dried to give cream coloured Ugi adduct (650 mg, 87%). m.p. 216-219oC, Rf(50%EtOAc/n-hexane): 0.50.
Full Article
SyntheticPage 797
Published Aug 25, 2015
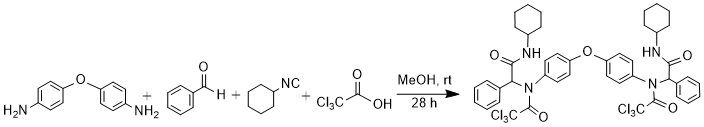
Procedure
A solution of 4,4'-diaminodiphenylether (0.998 mmol, 200 mg) in MeOH (7 mL) was treated with benzaldehyde (1.99 mmol, 0.20 mL), a solution of cyclohexyl isocyanide (1.99 mmol, 0.24 mL) in MeOH (3 mL), and trichloroacetic acid (1.99 mmol, 326 mg) in the order given. The reaction mixture was stirred at room temperature for 28 hours, and then filtered. The precipitate was washed with first Et2O and then with n-hexane to remove unreacted reagents or by-products and dried to give white Ugi adduct (640 mg, 70%). m.p. 248-251oC, Rf(50%EtOAc/n-hexane): 0.82
Full Article
SyntheticPage 798
Published Aug 25, 2015
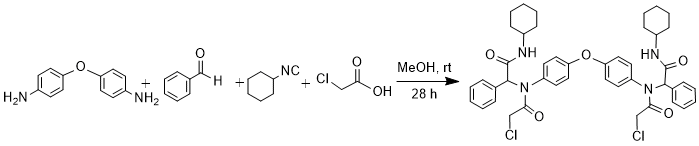
Procedure
A solution of 4,4'-diaminodiphenylether (0.998 mmol, 200 mg) in MeOH (7 mL) was treated with benzaldehyde (1.99 mmol, 0.20 mL), a solution of cyclohexyl isocyanide (1.99 mmol, 0.24 mL) in MeOH (3 mL), and chloroacetic acid (1.99 mmol, 188 mg) in the order given. The reaction mixture was stirred at room temperature for 28 hours, and then filtered. The precipitate was washed with first Et2O and then with n-hexane to remove unreacted reagents or by-products and dried to give white Ugi adduct (720 mg, 92%). m.p. 192-195oC, Rf(80%EtOAc/n-hexane): 0.81
Full Article
SyntheticPage 826
Published Jun 21, 2017
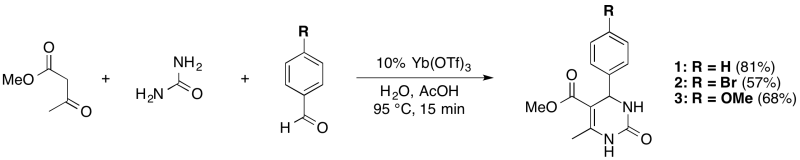
Procedure
Urea (0.48 g, 8.0 mmol) and ytterbium(III) triflate (0.26 g, 0.4 mmol) were added to a 1 dram (3.7 mL) glass reaction vial. Then, the 3:1 acetic acid:water solvent mixture (2 mL) was added to the vial; next, the methyl acetoacetate (0.86 mL, 8 mmol) was added, followed by the aldehyde (4 mmol). The stirred solution was heated for 15 minutes at 95 °C and developed an intense yellow colour. The reaction vial was cooled to room temperature; and, its contents were poured into a 25 mL beaker with 2 mL of ice and 5 mL of deionized water to fully precipitate the product. The solid was collected by vacuum filtration using a hirsch filter. The solid was washed with cold water (5 mL), then with toluene (5 mL); removal of the residual solvent by rotary evaporation provided the product in reasonable purity.
Full Article
 Substitution reaction of
Substitution reaction of