SyntheticPage 33
Published Jul 16, 2001
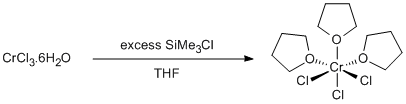
Procedure
Chromium(III) chloride hexahydrate (2.66 g, 10 mmol) and THF (20 ml) were placed in a three-neck 100 ml round bottomed flask equipped with a water condenser and large teflon stir-bar. The system was flushed with argon. Trimethylchlorosilane (32 ml, 253 mmol) was added dropwise with stirring to the slurry. The colour of the reaction mixture changed from dark green to deep purple. The reaction mixture was heated to reflux overnight (15 h) over which time the solution turned a lighter purple. On standing and cooling a purple solid precipitated from the solution. This was collected on an inert gas frit and washed with 40/60. Alternatively the solution could be decanted off and the solid washed in situ. The solid was dried in vacuo. Yield = 3.14 g, 84 %.
Full Article
SyntheticPage 505
Published Sep 05, 2011
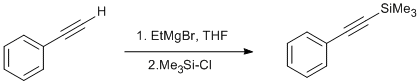
Procedure
To a flamed dried 250 mL one-neck round bottom flask equipped with stirbar, septa, and nitrogen inlet needle was added phenylacetylene (4.1 g, 0.040 mol, 1 equiv.) in THF (25 mL, 1.6 M in the alkyne) via a gastight syringe. The flask was cooled to -78 oC in a dry-ice/acetone bath. After cooling for 10 minutes, EtMgBr (60 mL, 0.060 mol, 1.5 equiv.) was added dropwise to the solution. Upon completion of addition, the mixture was stirred for 30 min. The solution was then warmed to 0 oC in a ice-water bath and stirred for 30 min. TMS-Cl (4.35 g, 0.040 mol, 1 equiv.) was added to the solution dropwise. The solution was stirred for 10 min after complete addition of TMS-Cl.. The ice-water bath was then removed and the solution was allowed to warm to room temperature for 30 min. The reaction mixture was carefully quenched1 by the addition of 60 mL of 1 M HCL (CAUTION: Exothermic). Upon complete addition, the biphasic mixture was transferred to a 1000 mL separatory funnel. The phases were separated and the aqueous layer was extracted with hexanes (3 x 100 mL). The combine organic layers were washed with brine and dried with Na2SO4. The solvent was removed in vacuo to afford a crude yellow-orange oil. Further purification was accomplished by vacuum distillation ( 57-61 oC @ 1 mmHg) to give pure trimethyl(phenylethynyl)silane (5.65 g, 81%).
Full Article
SyntheticPage 653
Published Jul 25, 2013
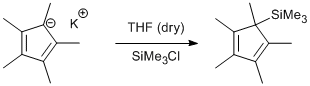
Procedure
KCp* (1.0 g, 5.7 mmol) was suspended in THF (30 mL) and cooled to 0 °C. Trimethylsilyl chloride (0.74 mL, 7.6 mmol) was added drop wise and the resulting mixture was stirred overnight at room temperature. The resulting suspension was filtered through celite and the solvent was removed in vacuo to give the product as a yellow oil (Yield 0.79 g, 71 %).
Full Article
 Dehydration of chromium(III) chloride
Dehydration of chromium(III) chloride