|
23/03/2013 18:15:48 |
|
Normal
|
|
Acknowledged
|
|
|
|
|
|
22/04/2013 16:29:02 |
|
Thank you for your feedback.
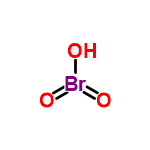
The pH will depend on the pKa of your acid (which is -2 for Bromic Acid), along with the solvent and the concentration. This is not the type of information ChemSpider covers, and I suggest you look in your chemistry textbook for more information.
|